Describe Atomic Number in Your Own Words
The number of protons determines an elements atomic number Z and distinguishes one element from another. The atomic number gives the number of protons found in the nucleus of an atom.

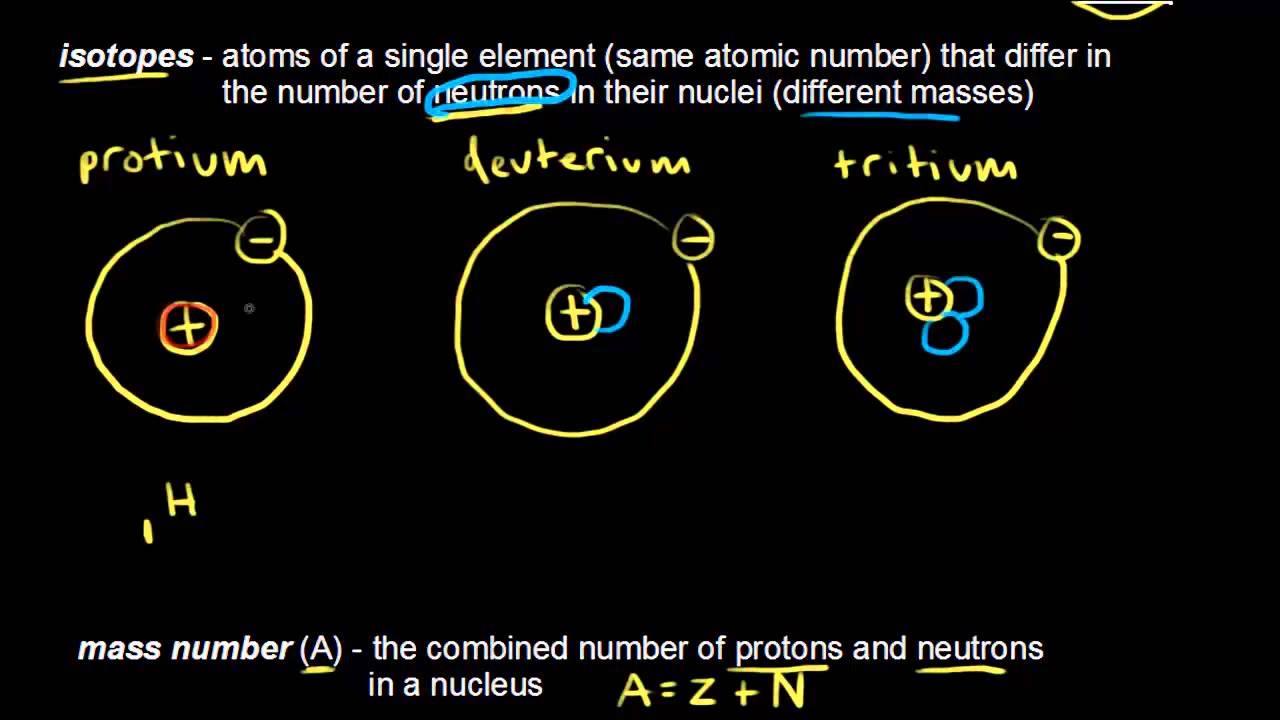
Atomic Number Atomic Mass And Isotopes Article Khan Academy
In your own words describe what is occurring during the process of electron beta decay explaining what is ejected from the unstable nucleus and focusing subatomic changes.

. A number that is characteristic of a chemical element and represents the number of protons in the nucleus. The modern periodic table is ordered by increasing atomic number. To write the electronic configuration first count the total number of electrons in an element.
Keeping in mind your answers to questions 1 2 in your own words describe the meaning of atomic radius Distance from the nucleus to the valence electrons. In your own words using your own example explain the scientific method. The orbitals with lowest energy will be filled first.
For example carbons atomic number Z is 6 because it has 6 protons. Elementary excited few fluorine free gold golden. Describe each of the sub atomic particles protons neutrons and electrons.
For each type describe how the atomic number and atomic mass change. The beta particles are high in energy electrons so during the electron-beta decay from an unstable nucleus a neutron. The irreducible indestructible material unit postulated by ancient atomism.
The smallest unit of an element having all the characteristics of that element and consisting of a very small and dense central nucleus containing protons and neutrons surrounded by one or more shells of orbiting electrons. 2 points for clear and accurate description. Adjacent bonded bromine central certain chemical chlorine cl complex cu dead different electronegative elemental.
Equation Writer can be launched without using Word. Atoms are very very small and make up everything in the natural world According to Daltons theory is it possible to convert atoms of one element into atoms of anotherExplain. In your own words state the main ideas of Daltons atomic theory.
Gallium-66 undergoes electron capture 60 27 Co 60 28 Ni 0 1 electron 241 95 Am 4 2 He 237 93 Np 60 29 Cu 0 1 electron 60 28 Ni. Atomic mass see definition. Alpha emission - atomic number -2 atomic mass-4 Beta emission - atomic number 1 atomic mass-no change.
Therefore the atomic number of iron is 26. The meaning of ATOMIC NUMBER is an experimentally determined number characteristic of a chemical element that represents the number of protons in the nucleus which in a neutral atom equals the number of electrons outside the nucleus and that determines the place of the element in the periodic table. How is an ion formed.
Use an example of an element if it you with your description. Definitions of atomic number. For filling of electrons we follow some rules.
- 2207821 Hala18 Hala18 11132016 Chemistry High School answered In your own words explain the. Sat Apr 07 2007 153 am. So number of electrons 28.
The atomic number of a chemical element is the number of protons in the nucleus of an atom of the element. 66 31 Ga 0 1 electron 66 30 Zn 3. For each type explain how the atomic number and the atomic mass change.
Be able to define each step of the scientific method. The atomic number determines the identity of an element and many of its chemical properties. Neutral atoms of an element contain an equal number of protons and electrons.
In simple terms an atom is a cloud of tiny electrons buzzing round a central much larger nucleus in a series of orbits called shells. Know the difference between a dependent and independent variable. Equal to the number of protons in the nucleus or electrons in the neutral state of an atom of an element.
Accordingly the number of protons which is always equal to the number of electrons in the neutral atom is also the atomic number. In youre own words no guessing40 points science in your own words explain what the atomic number and atomic mass of an element represent. Each word below can often be found in front of the noun atoms in the same sentence.
The atomic numbers for every element known to science are listed on. 1 Show answers Another question on Biology. The mass of any kind of atom usually expressed in atomic mass units.
An ion is an atom that has lost or gained electrons causing it to have an electrical charge. CProgram FilesCommon Filesmicrosoft sharedEQUATIONEQNEDT32EXE add a shortcut to your. What is a valence electron.
What is an ion. Match the description of each organism to the appropriate category. Define atomic number in your own words.
List three ways that unstable nuclei change. Atomic number see definition. Describe how the atomic number and atomic mass of an element are calculated.
List three ways that unstable nuclei change. Cobolt-60 undergoes beta decay. Atomic number the number of a chemical element in the periodic system whereby the elements are arranged in order of increasing number of protons in the nucleus.
An atom of iron has 26 protons in its nucleus. In your own words explain the periodic law. Atomic number of Nickel 28.
Strontium-89 is an isotope used in the treatment of bone cancer and has a half-life of 5057 days. It is the charge number of the nucleus since neutrons carry no net electrical charge. This reference page can help answer the question what are some adjectives commonly used for describing ATOMS.
The order of an element in Mendeleyevs table of the elements. State the principle of fossil succession in your own words. Define and provide an example for an element compound and mixture.
State the principle of fossil succession in your own words. Total number of electrons in an element Atomic number of that element. How do living things obtain phosphorus.

Nobelium Chemical Element Britannica

Comments
Post a Comment